Resource Highlights
We want you to have all of the tools you need throughout the Fensolvi prescribing process.
The resources below will help you enhance your expertise and improve patient outcomes.
Patient Enrollment Form
Download the patient enrollment form to get your patient started on Fensolvi.


Letter of Medical Necessity
Learn tips for composing a letter of medical necessity.
Mixing & Administration
View our mixing & administration guide and video.
Patient Support
Learn about ways you can support your patients throughout their Fensolvi journey.
Clinical Info & FAQs
Innovative technology in the delivery of leuprolide acetate
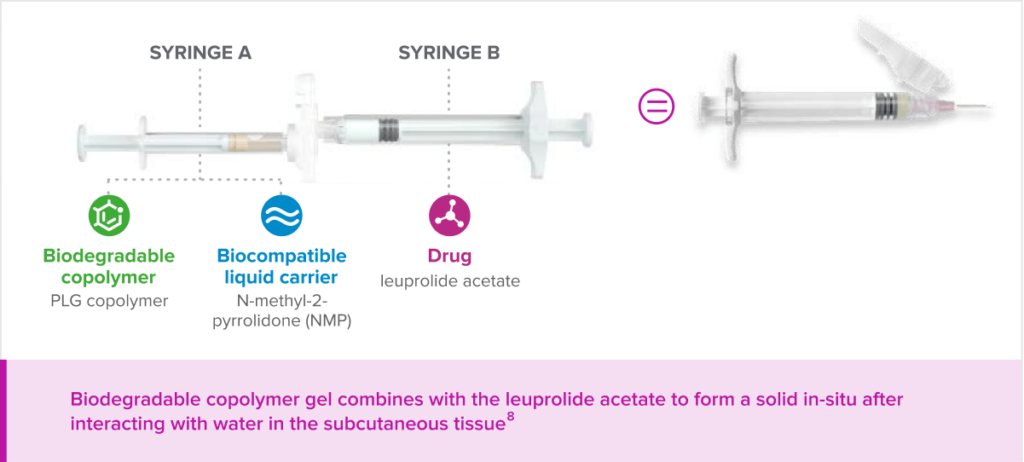
Fensolvi (leuprolide acetate) for injectable suspension is the first and only extended-release subcutaneously administered leuprolide acetate formulation for this indication. It uses a biodegradable gel delivery system, which contains a unique polymer of poly(DL-lactide-co-glycolide), dissolved in N-methyl-2-pyrrolidone. After injection, the polymeric gel suspension delivery system forms an in-situ solid, enabling sustained and controlled release of leuprolide acetate as it safely biodegrades.
Absorption occurs in two phases, a burst phase followed by a plateau phase. The mean plateau serum leuprolide level from 4 to 48 weeks was approximately 0.37 ng/mL with a range of 0.18 to 0.63 ng/mL.
Figure 4. SC injection
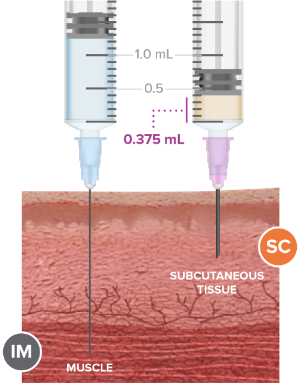
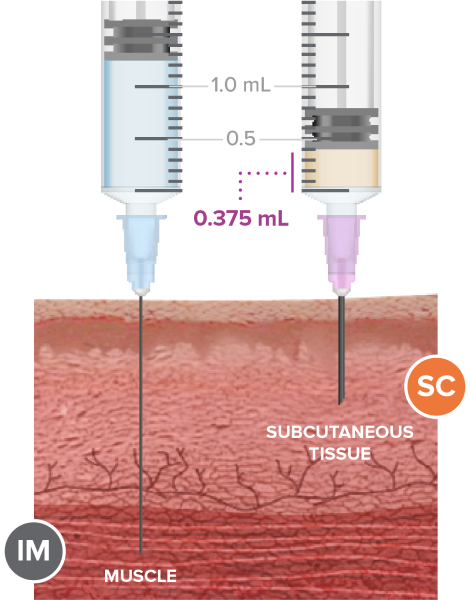
The benefits of subcutaneous injections (Figure 4) for healthcare providers and patients (3, 6, 7):
- Lack of “the-day-after” local muscle pain typically associated with IM injection
- Little muscle mass (common among children) is not a concern
- Reduced risk of hitting bone or nerve
- Flexibility of injection sites
- No surgery or product removal required
Full prescribing information can be found here
Fensolvi® delivers leuprolide acetate through a novel, in-situ polymeric gel extended delivery system.
A single subcutaneous injection delivers 6 months of treatment.
The novel delivery releases leuprolide acetate slowly over time as the copolymer safely biodegrades.
Fensolvi remains in-situ and slowly biodegrades over the intended 6-month dosing interval.
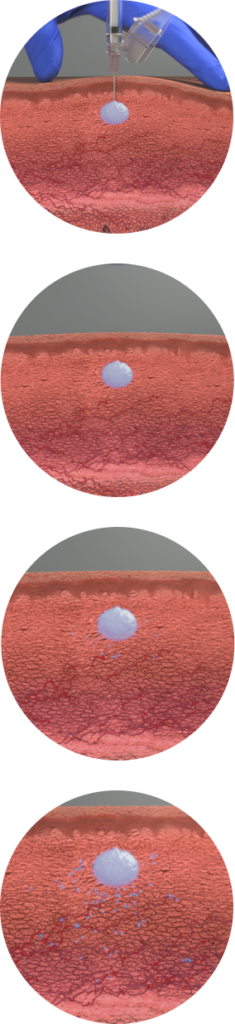
Fensolvi® delivers leuprolide acetate through a novel, in-situ polymeric gel extended delivery system.
Biodegradeable copolymer gel combines with leuprolide acetate to form a solid in-situ after interacting with water in the subcutaneous tissue.
Class: Fensolvi (leuprolide acetate) for injectable suspension, is the only subcutaneously delivered leuprolide acetate in the class of Gonadotropin Releasing Hormone (GnRH) agonists.
Indication: Fensolvi is indicated for the treatment of pediatric patients 2 years of age and older with central precocious puberty.
THERE ARE 2 EASY WAYS TO ORDER FENSOLVI®
1. Use your current eRx platform
Send a prescription directly to Fensolvi TotalSolutions® via the Scripts Rx Pharmacy
Scripts Rx:
1815 S. Meyers Rd. Suite 100
Oakbrook Terrace, IL 60181
7515 Main St. Suite 180
Houston, TX 77030
NPI: 1144730995
NABP Number: 5922592
Phone: 833-213-9520
Fax: 877-991-1798
or
2. Fax patient enrollment form
Fax the completed patient enrollment form to Fensolvi TotalSolutions®
Fax: 877-991-1798
Click here to download the Fensolvi TotalSolutions Patient Enrollment Form.
Fensolvi is a refrigerated product. However, once removed from refrigeration, it can be stored at room temperature (59-86°F) in original packaging for up to 8 weeks.(1)
| Coding Information for FENSOLVI | |
| NDC for FENSOLVI | 62935-0163-60 (for billing purposes) |
| HCPCS Code for FENSOLVI | J1951 |
| J-code descriptions | Injection, leuprolide acetate for depot suspension, 0.25 mg |
| Billable Unit | 0.25 mg |
| Units | 180 |
CMS has established a new, permanent Healthcare Common Procedure Coding System (HCPCS) code for FENSOLVI® (leuprolide acetate) for injectable suspension, effective July 1, 2021.
Tolmar does not guarantee coverage or payment. The healthcare provider should follow all billing and coding requirements established by the insurance company to submit compliant claims for FENSOLVI. All codes on the claim form should be supported by the documentation in the patient’s medical record.
Learn more about how this may impact your current billing practices here.
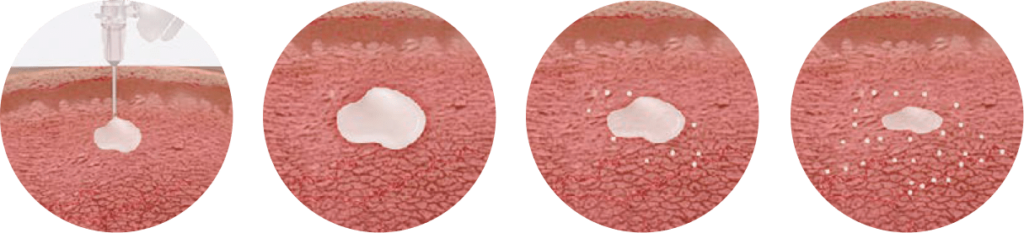
Simple steps for preparation and injection
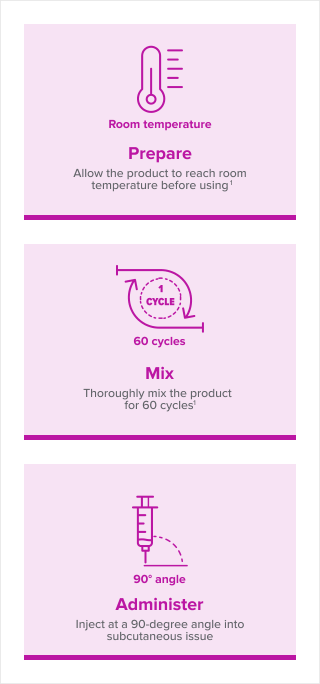
For complete mixing and administration instructions, view this video or download this detailed guide.
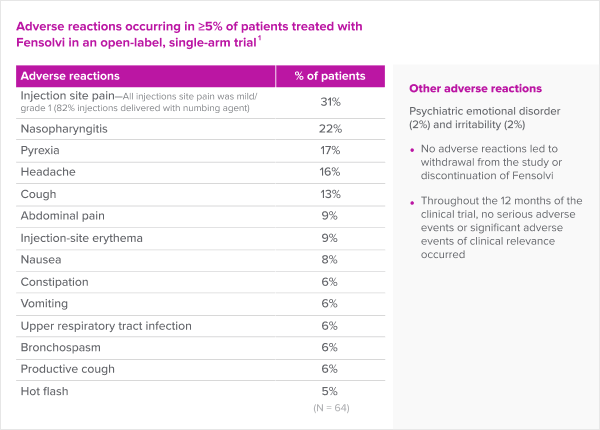
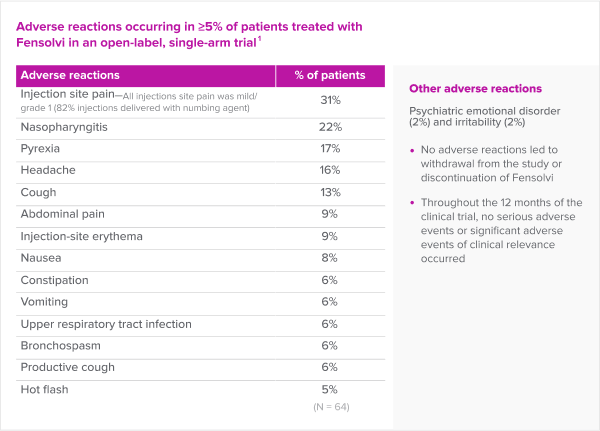
Fensolvi has a favorable safety and tolerability profile.
- No adverse reactions led to withdrawal from the study or discontinuation of Fensolvi
- Throughout the 12 months of the clinical trial, no serious adverse reactions or significant adverse reactions of clinical relevance occurred
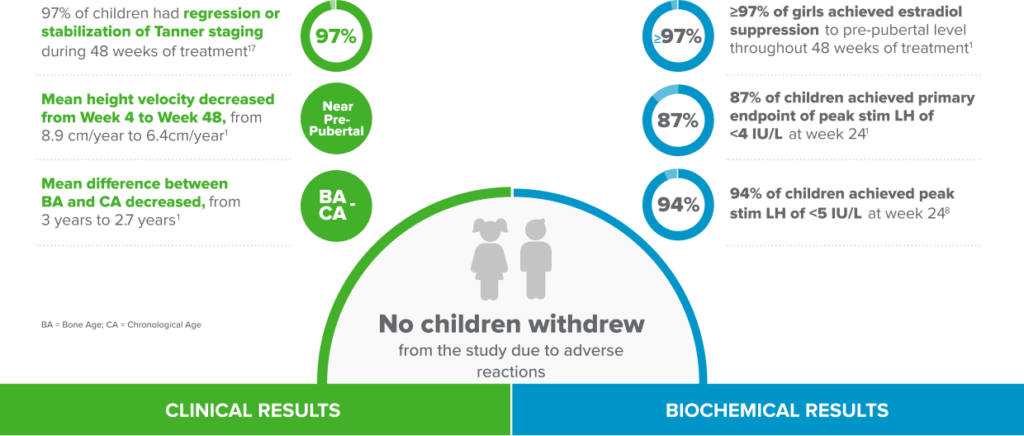
Fensolvi clinically demonstrated sustained suppression of stimulated luteinizing hormone (LH) level to <4 IU/L1.
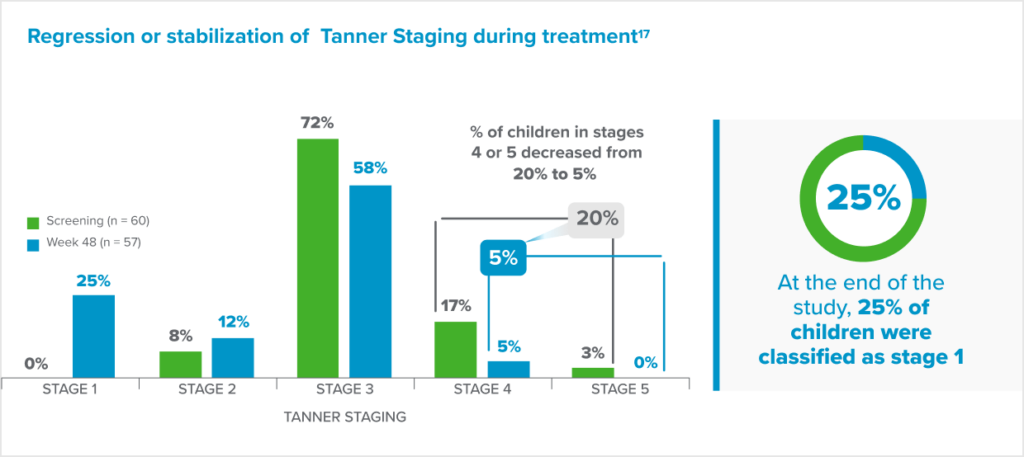
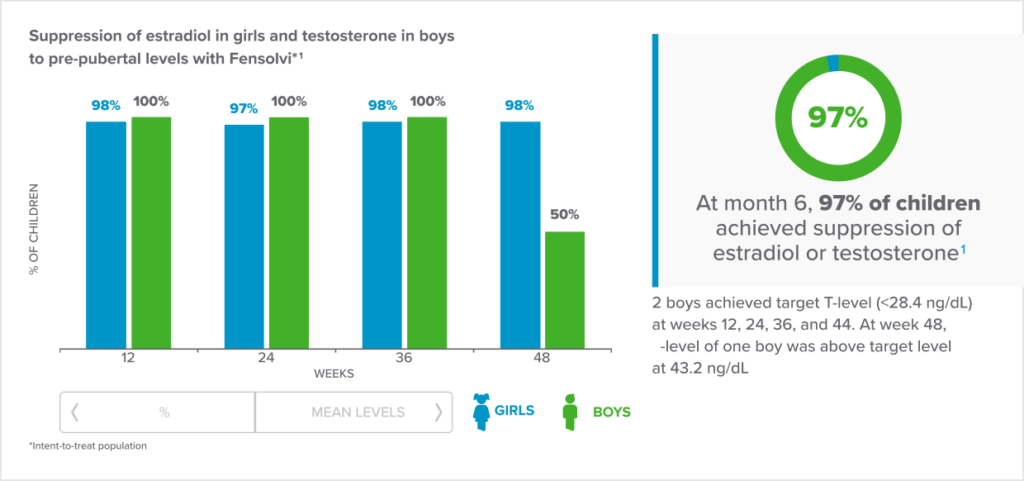
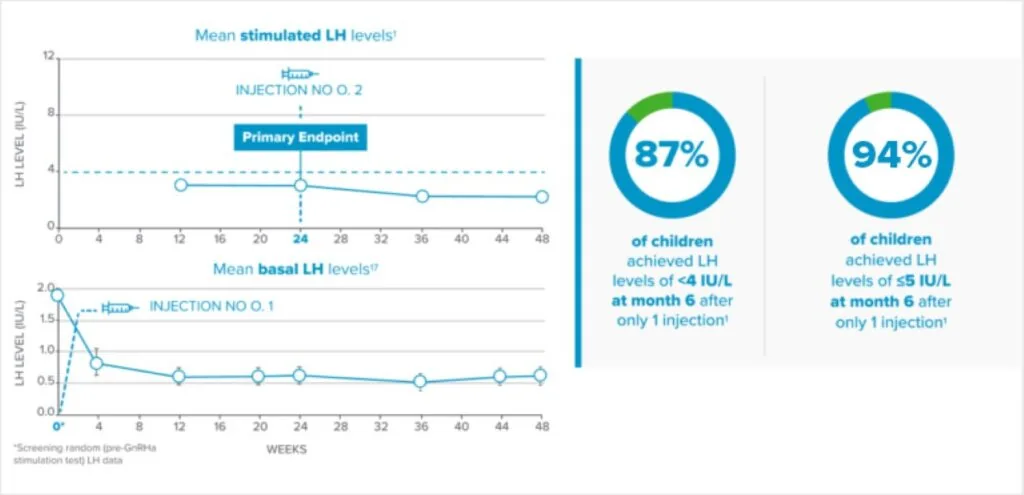
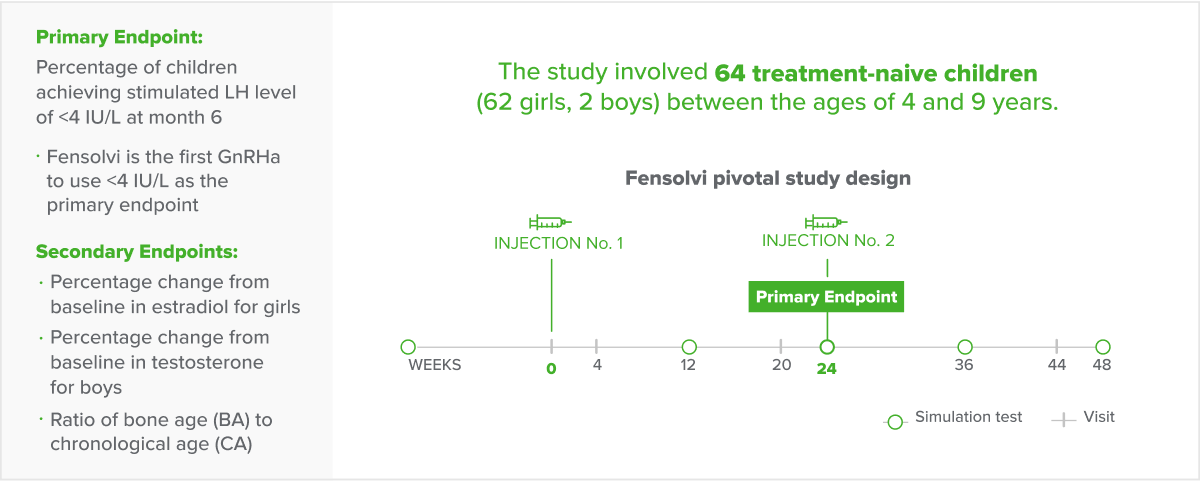
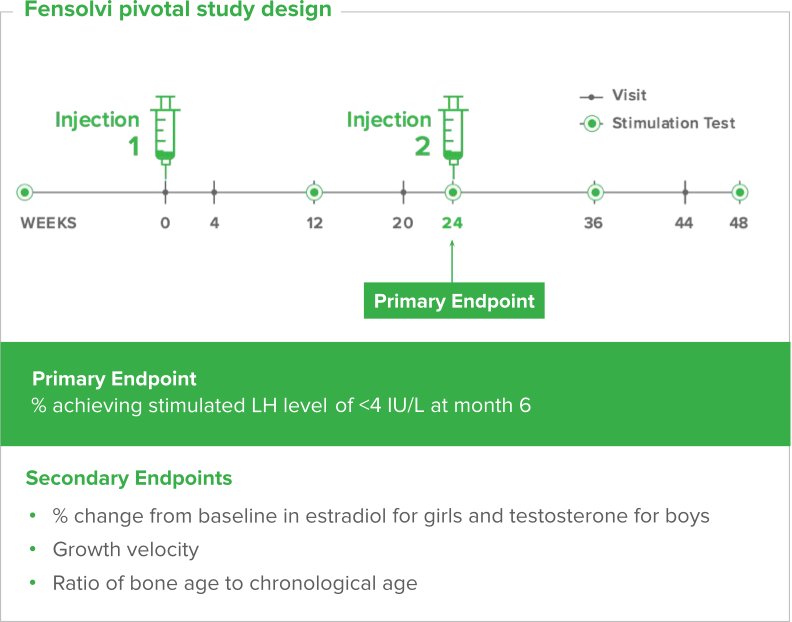
Fensolvi efficacy was demonstrated through a 12-month, uncontrolled, open-label, single-arm clinical trial. The clinical trial included 64 children with CPP (62 girls and 2 boys, 4–9 years old) who were naïve to GnRH agonist treatment.
Fensolvi 45 mg subcutaneous administered every 6 months was well tolerated. No adverse reactions led to withdrawal from the study or discontinuation of study drug. Most treatment-emergent adverse reactions were Grade 1 or 2.(2)
To report suspected adverse reactions contact Tolmar at 1-844-4TOLMAR (486-5627) or the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
Fensolvi TotalSolutions keeps things simple for your practice, your patients, and their parents.
We offer a full line of patient support services, including:
- Benefit verification
- Assistance with prior authorizations, appeals, and billing and coding inquiries
- Patient education materials
- Copay Assistance Program
- Patient Assistance Program
You can reach Fensolvi TotalSolutions at 1-866-FENSOLVI (336-7658) or fensolvitotalsolutions.com.
All patient-friendly resources can be found here.
USEFUL DOCUMENTS
Explore our resources to learn how Fensolvi can help you keep childhood simple for children with CPP. To access patient resources in English and Spanish, click here.

Mixing and Administration
Watch the video to view mixing and administration instructions.
For full dosage and administration guidelines, click here.
Your patients and their families can stay connected to their treatment and your practice through dedicated patient support with Fensolvi TotalSolutions.
You can reach Fensolvi TotalSolutions at
1‑866‑FENSOLVI (336‑7658) or fensolvitotalsolutions.com
We offer a full line of patient support services, including:
With our innovative online portal, you can receive the results of benefits investigations in an average of 4 hours, including both pharmacy and medical claims
Fensolvi TotalSolutions can provide prior authorization information and support, including appeal support
The Fensolvi TotalSolutions concierge team is ready to help answer any billing or coding questions you may have
Fensolvi TotalSolutions concierges will help transfer your patients prescription to a dedicated specialty pharmacy
Fensolvi TotalSolutions offers copay assistance for your eligible patients, allowing them to pay as little as $5 for their Prescription.
See terms and conditions for more information

Fensolvi Starter Kits
We’re excited to offer Fensolvi starter kits for new patients who have been prescribed Fensolvi. Each kit offers helpful information for caregivers plus engaging activities for children to enjoy during their visits. Contents include a treatment brochure, coloring and iSpy sheets, coloring pencils, a book of jokes, and coping cards—all inside a handy drawstring bag. Order kits for your patients by calling your Tolmar representative or completing the form below.
Join Our Live Virtual M&A Demonstrations
Virtual Training Sessions include a live demonstration on mixing and administering Fensolvi. The clinical trainer will answer any questions you may have.
Live in-service training with your local Account Manager in person is encouraged and can be requested in the next section “Request: In-Person M&A Training”.
Connect with Fensolvi
Connect with a Fensolvi Representative
By providing your information, you are giving Tolmar, Inc. and other parties working with us permission to communicate with you about Fensolvi® or other products, services, and offers from Tolmar, Inc. If you don’t find the information useful, you can opt out at any time. We value your privacy and we encourage you to review our privacy policy for more information.
Contact us at 1-866-FENSOLVI (336-7658)
"*" indicates required fields
Fensolvi Starter Kits
We’re excited to offer Fensolvi starter kits for new patients who have been prescribed Fensolvi. Each kit offers helpful information for caregivers plus engaging activities for children to enjoy during their visits. Contents include a treatment brochure, coloring and iSpy sheets, coloring pencils, a book of jokes, and coping cards—all inside a handy drawstring bag. Order kits for your patients by calling your Tolmar representative or completing the form.
Contact us at 1-866-FENSOLVI (336-7658)
"*" indicates required fields
Contact us to schedule a mixing and administration demonstration
We will only use your submission to communicate with you about scheduling a mixing and administration training and to share associated materials. Please see our privacy policy for more information.
Contact us at 1-866-FENSOLVI (336-7658)
"*" indicates required fields
Sign up to receive clinical and support updates
Complete the form below to receive emails featuring key updates related to Fensolvi. By providing your information, you are giving Tolmar, Inc. and other parties working with us permission to communicate with you about Fensolvi® or other products, services, and offers from Tolmar, Inc. If you don’t find the information useful, you can opt out at any time. We value your privacy and we encourage you to review our privacy policy for more information.
Contact us at 1-866-FENSOLVI (336-7658)
"*" indicates required fields
IMPORTANT SAFETY INFORMATION
FENSOLVI (leuprolide acetate) for injectable suspension is a gonadotropin releasing hormone (GnRH) agonist used to treat patients 2 years of age and older with central precocious puberty (CPP). CPP may be diagnosed when signs of sexual maturity begin to develop in girls under the age of 8 or in boys under the age of 9.
FENSOLVI is contraindicated in individuals with hypersensitivity to any drug that is in the same class as FENSOLVI, in individuals who are allergic to any of the ingredients in FENSOLVI, or in individuals who are pregnant. FENSOLVI may cause fetal harm when administered to a pregnant patient.
During the first few weeks of treatment, increases in gonadotropins and sex steroids above baseline may result in an increase in signs and symptoms of puberty including vaginal bleeding in girls.
Psychiatric events have been reported in patients taking GnRH agonists. Events include emotional lability, such as crying, irritability, impatience, anger, and aggression. Patients should be monitored for development or worsening of psychiatric symptoms.
Convulsions have been observed in patients treated with GnRH agonists with or without a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs.
Pseudotumor Cerebri (Idiopathic Intracranial Hypertension) has been reported in pediatric patients treated with GnRH agonists. Patients should be monitored for headache, papilledema and blurred vision.
The most common adverse reactions seen with FENSOLVI were: injection site pain, nasopharyngitis, pyrexia, headache, cough, abdominal pain, injection site erythema, nausea, constipation, vomiting, upper respiratory tract infection, bronchospasm, productive cough and hot flush.
Please see Full Prescribing Information for FENSOLVI for additional important safety information.
To report suspected adverse reactions contact Tolmar at 1-844-4TOLMAR (486-5627) or the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
IMPORTANT SAFETY INFORMATION
FENSOLVI (leuprolide acetate) for injectable suspension is a gonadotropin releasing hormone (GnRH) agonist used to treat patients 2 years of age and older with central precocious puberty (CPP). CPP may be diagnosed when signs of sexual maturity begin to develop in girls under the age of 8 or in boys under the age of 9.
FENSOLVI is contraindicated in individuals with hypersensitivity to any drug that is in the same class as FENSOLVI, in individuals who are allergic to any of the ingredients in FENSOLVI, or in individuals who are pregnant. FENSOLVI may cause fetal harm when administered to a pregnant patient.
During the first few weeks of treatment, increases in gonadotropins and sex steroids above baseline may result in an increase in signs and symptoms of puberty including vaginal bleeding in girls.
Psychiatric events have been reported in patients taking GnRH agonists. Events include emotional lability, such as crying, irritability, impatience, anger, and aggression. Patients should be monitored for development or worsening of psychiatric symptoms.
Convulsions have been observed in patients treated with GnRH agonists with or without a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs.
Pseudotumor Cerebri (Idiopathic Intracranial Hypertension) has been reported in pediatric patients treated with GnRH agonists. Patients should be monitored for headache, papilledema and blurred vision.
The most common adverse reactions seen with FENSOLVI were: injection site pain, nasopharyngitis, pyrexia, headache, cough, abdominal pain, injection site erythema, nausea, constipation, vomiting, upper respiratory tract infection, bronchospasm, productive cough and hot flush.
Please see Full Prescribing Information for FENSOLVI for additional important safety information.
To report suspected adverse reactions contact Tolmar at 1-844-4TOLMAR (486-5627) or the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
References
- Fensolvi® (leuprolide acetate) for injectable suspension 45 mg Prescribing Information. Dublin 2, Ireland: Tolmar International, Ltd.; 11/2022.
- Klein K, Freire A, Gryngarten M, et al. Phase 3 trial of a small-volume subcutaneous 6-month duration leuprolide acetate treatment for central precocious puberty. J Clin Endo Metab. 2020;105(10):1-12.
- Prettyman J, Engel L, Boldt-Houle D, et al. Personalizing treatment in the delivery of care by nurses to patients with prostate cancer. Urologic Nursing. 2019;39(2):83-99
- LUPRON DEPOT-PED [package insert]. North Chicago, IL: AbbVie Inc.
- Triptodur [package insert]. Atlanta, GA: Arbor Pharmaceuticals, LLC.
- Russo LG, Moore WV. 444 Comparison of intramuscular and subcutaneous injections of growth hormone. Pediatric Research 1981 15(4) 514
- Leung AK, Chiu AS, Siu TO. Subcutaneous versus intramuscular administration of Haemophilus influenzae type b vaccine. J R Soc Health. 1989;109(2):71-73.
- Sartor O. A new form of treatment for prostate cancer. European Urology Supplements. 2006;5:905-910.
FENSOLVI TOTALSOLUTIONS COPAY PROGRAM TERMS AND CONDITIONS
The Fensolvi® Co-pay Assistance Program (“Program”) is valid ONLY for patients who are prescribed Fensolvi® and are reimbursed exclusively by commercial insurance. This Program is valid only in the United States; but, void where prohibited by law or by the patient’s health insurance provider. This Program is non-transferable, limited to one per person, and cannot be combined with any other coupon, free trial, discount, prescription savings card, or other offer. Restrictions or limits may apply.
Medicare, Medicaid, Tricare and other federal health care program beneficiaries may not participate in this Program. This Program also is neither available for cash paying patients nor where your commercial plan reimburses you for the entire cost of your prescription drug. Patients cannot seek reimbursement from health insurance or any third party for any part of the assistance received through this Program. The patient or his/her guardian is responsible for reporting the receipt of all benefits or reimbursement received under the Program to any insurer, health plan, or other third party, as may be required. This Program is not insurance and is not intended as a substitute for insurance.
With the Program, you pay as little as $5 of your co-pay or co-insurance for Fensolvi®, per prescription. The remainder of your co-pay or co-insurance is covered, up to two prescriptions per calendar year. The Program assists with the cost of Fensolvi only. It does not assist with the cost of other administrations, medicines, procedures or office visit fees.
Tolmar, Inc. (“Tolmar”) reserves the right to terminate, rescind, revoke, or modify this Program at any time without notice. This Program expires at the end of the current calendar year, at which time you must re-enroll. For complete information about the terms and conditions of this Program, including the limitations on use and the amount of assistance call 1 866-FENSOLVI (336-7658).
Program managed by Scripts Rx on behalf of Tolmar.